Poly(vinylidene fluoride) (PVDF) is a semi‐crystalline fluoropolymer known for its exceptional chemical resistance, mechanical strength, and piezoelectric properties.
Within PVDF materials, two primary categories exist: homopolymer PVDF and copolymer PVDF. While both share a vinylidene fluoride (–CH₂–CF₂–) backbone, the inclusion of secondary monomers in copolymers modifies its crystallinity, thermal behavior, mechanical flexibility, and processability.
This article outlines the fundamental differences between homopolymer and copolymer PVDF, covering their chemical structures, properties, processing considerations, and typical applications.
1. Chemical Structure and Synthesis
1.1 Homopolymer PVDF
- Monomer Composition
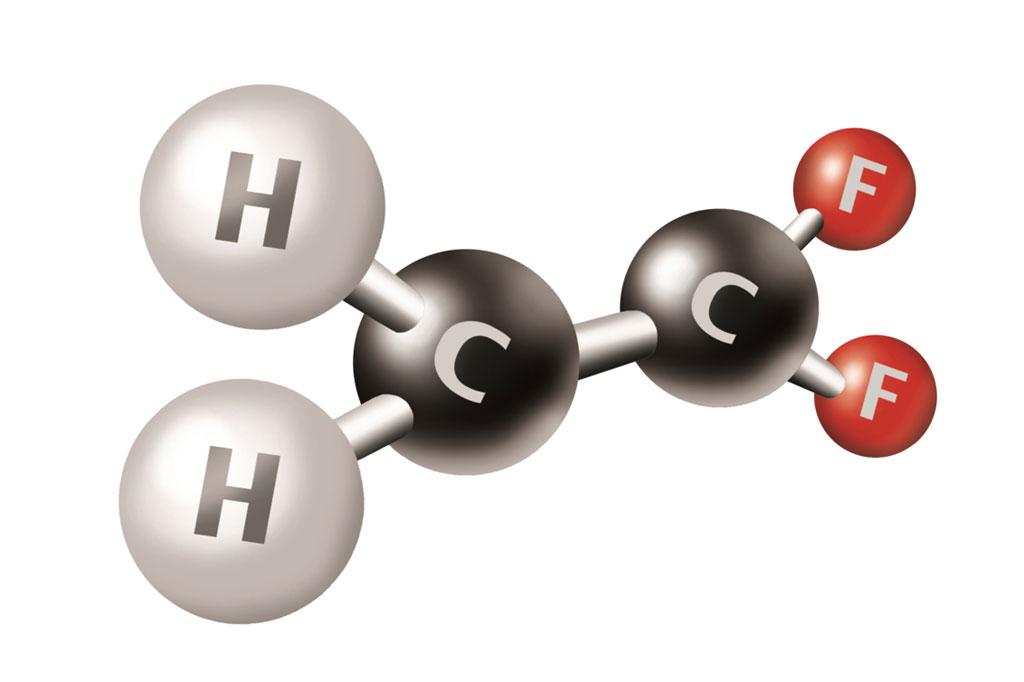
- Consists solely of vinylidene fluoride (VDF) monomer units (–CH₂–CF₂–)_n
- Polymerization
- Typically produced via suspension or emulsion polymerization of VDF under controlled temperature and pressure.
- Yields a highly regular chain sequence, enabling tight chain packing and high crystallinity.
1.2 Copolymer PVDF
- Monomer Composition
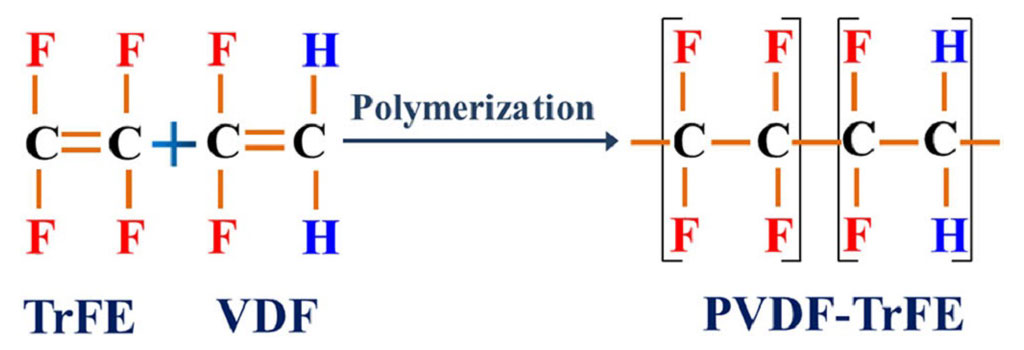
- Incorporates a second monomer—most commonly hexafluoropropylene (HFP) or trifluoroethylene (TrFE)—randomly or in blocks along with VDF.
- Two principal copolymer families:
- PVDF-HFP (VDF/HFP copolymer)
- VDF-TrFE (VDF/TrFE copolymer)
- Polymerization
- Conducted similarly to homopolymer PVDF, but with controlled feed ratios of VDF and the comonomer.
- Random insertion of HFP or TrFE disrupts the perfect PVDF chain regularity, reducing crystallinity and lowering melting point.
2. Crystallinity and Thermal Properties
2.1 Degree of Crystallinity
- Homopolymer PVDF
- High crystallinity (typically 45–55% by weight).
- Distinct crystalline β-phase can be induced through stretching or electrical poling, contributing to strong piezoelectric response.
- Copolymer PVDF
- Reduced crystallinity (often 30–40% or lower, depending on comonomer content).
- Incorporation of HFP or TrFE disrupts chain packing, creating more amorphous regions.
2.2 Melting Point (Tₘ) and Glass Transition (Tg)
- Homopolymer PVDF
- Tₘ around 170 °C (often ranges 165–175 °C depending on molecular weight and processing conditions).
- Tg is approximately –35 °C.
- PVDF-HFP Copolymer
- Lower Tₘ in proportion to HFP content; typical Tₘ ranges 140–160 °C (HFP content 8–20%).
- Tg shifts slightly upward (around –20 °C to –15 °C) due to increased chain mobility in amorphous regions.
- VDF-TrFE Copolymer
- For near‐equimolar compositions (e.g., VDF: TrFE ≈ 70:30 mol %), a ferroelectric Curie transition appears around 100 °C–120 °C.
- Melting peaks vary from 135 °C to 160 °C depending on the TrFE fraction.
- Tg moves closer to room temperature (≈ −10 °C to 0 °C) with increasing TrFE.
3. Mechanical Properties
3.1 Tensile Strength and Modulus
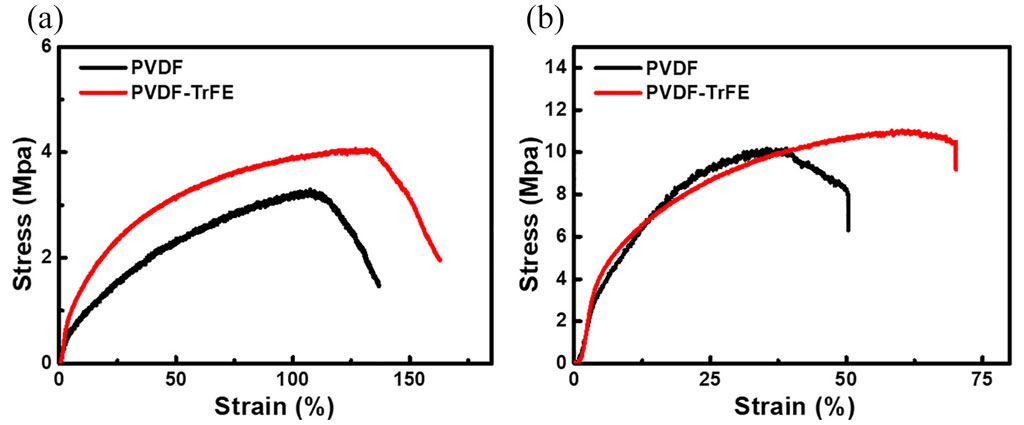
- Homopolymer PVDF
- Tensile strength: ~35–50 MPa
- Young’s modulus: ~1.5–2.2 GPa
- More rigid due to high crystallinity; lower elongation at break (≈ 50–100%).
- PVDF-HFP Copolymer
- Tensile strength: ~20–35 MPa (depending on HFP content)
- Modulus: ~0.8–1.5 GPa
- Increased elongation at break (up to 150–200%), making it more flexible.
- VDF-TrFE Copolymer
- Mechanical properties depend heavily on ratio and processing; often intermediate between homopolymer and PVDF-HFP.
- Good flexibility with elongation at break in the 100–150% range.
3.2 Impact Resistance and Toughness
- Homopolymer PVDF
- Fair impact resistance, but may be prone to brittle failure at low temperatures due to higher crystallinity.
- Copolymer PVDF
- Both PVDF-HFP and VDF-TrFE exhibit improved impact resistance and toughness, especially at subzero temperatures, owing to a larger amorphous fraction.
4. Electrical and Piezoelectric Behavior
4.1 Homopolymer PVDF
- Piezoelectric Response
- Can be polarized to form β-phase crystals with strong piezoelectric (d₃₃ ≈ −30 pC/N) and pyroelectric properties.
- Widely used in sensors, actuators, and transducers.
- Dielectric Constant
- Relatively high dielectric constant (~8–12 at 1 kHz), enabling capacitive applications.
4.2 VDF-TrFE Copolymer
- Ferroelectric and Piezoelectric
- Exhibits spontaneous polarization even without mechanical stretching; displays ferroelectric hysteresis below the Curie temperature.
- Moderate piezoelectric coefficient (d₃₃ ≈ −15 to −20 pC/N).
- Often preferred for ferroelectric memory and non-volatile applications.
- PVDF-HFP Copolymer
- Does not exhibit strong ferroelectric behavior; piezoelectric activity is lower than homopolymer PVDF due to less β-phase content.
- Primarily used where flexibility and chemical resistance matter more than piezoelectric performance.
5. Chemical Resistance and Environmental Stability
5.1 Homopolymer PVDF
- Outstanding resistance to acids, bases, and organic solvents up to 150 °C.
- Exceptional resistance to UV and gamma radiation.
- Relatively insensitive to hydrolysis and biodegradation.
5.2 Copolymer PVDF
- PVDF-HFP
- Maintains excellent chemical resistance similar to homopolymer, though marginally reduced due to amorphous regions that may allow slightly increased permeability.
- Better stress‐cracking resistance in aggressive media.
- VDF-TrFE
- Chemical resistance remains strong, though TrFE segments can marginally reduce crystallinity‐driven impermeability.
- Still suitable for harsh chemical environments, albeit with slightly lower maximum service temperature.
6. Processing and Fabrication
6.1 Melt Processing
- Homopolymer PVDF
- Narrow processing window: requires precise temperature control (around 180–220 °C).
- Higher viscosity in the molten state; extrusion and injection molding demand heavier machinery.
- Copolymer PVDF
- PVDF-HFP: Lower melt viscosity and melting temperature (≈ 160–200 °C), facilitating easier extrusion, blow molding, and film casting.
- VDF-TrFE: Also exhibits lower melting point; readily processed into thin films by melt‐casting or solution‐casting.
- Additive Compatibility
- Copolymers accept plasticizers and fillers more readily, broadening composite options (e.g., carbon nanotube‐PVDF‐HFP for flexible batteries).
6.2 Solvent Casting and Coating
- Homopolymer PVDF
- Limited solubility: typically dissolved in polar aprotic solvents (e.g., N,N‐dimethylformamide, DMF; dimethylacetamide, DMAc; or N‐methylpyrrolidone, NMP).
- Thicker films (25–100 µm) are more straightforward; very thin films can be brittle if not stretched to induce β‐phase.
- Copolymer PVDF
- PVDF-HFP dissolves readily in a broader range of solvents, enabling spin‐coating, dip‐coating, and spray‐coating to form ultra‐thin films (< 10 µm).
- VDF-TrFE is also soluble in DMF and other polar aprotic solvents, allowing fabrication of high‐quality ferroelectric films for microelectronics.
7. Typical Applications
7.1 Homopolymer PVDF
- Wire and Cable Insulation: Superior electrical insulation, flame retardance, and weatherability.
- Chemical Processing Equipment: Linings, valves, and gaskets in corrosive environments (semiconductor etching, acid handling).
- Piezoelectric Sensors and Actuators: Ultrasonic transducers, strain gauges, microbalances, and energy harvesting devices.
- Membrane Filtration: Microfiltration and ultrafiltration membranes in water treatment, thanks to high hydrolytic stability.
7.2 PVDF-HFP Copolymer
- Flexible Battery Binders and Electrolytes: Porous structures ideal for lithium‐ion batteries and supercapacitors; combines mechanical flexibility with electrolyte wettability.
- Fuel Cell Membranes: Proton exchange membranes (PEMs) with enhanced ionic transport when blended with ionic liquids or Nafion.
- Coatings for Corrosion Protection: Thinner, conformal coatings on metal parts, offering chemical resistance with moderate flexibility.
- Dielectric Elastomers: Embedded in flexible electronics and sensors where compliance is crucial.
7.3 VDF-TrFE Copolymer
- Ferroelectric Memory (FRAM, FeRAM): Utilized as a dielectric layer exhibiting nonvolatile polarization switching capabilities.
- Infrared Detectors and Pyroelectric Sensors: Leveraging strong pyroelectric coefficients in thin‐film architectures.
- Electro-Active polymers (EAPs): Soft actuators, artificial muscles, and tunable optics.
- High‐Performance Piezoelectric Films: Although lower d₃₃ than homopolymer PVDF, easier to process into uniform ferroelectric layers.
8. Cost Considerations
- Homopolymer PVDF
- Generally more expensive on a per‐kilogram basis due to higher purity requirements and tighter process controls needed to achieve consistent β-phase content for piezoelectric applications.
- Copolymer PVDF
- PVDF-HFP: Typically lower cost than homopolymer PVDF, as the inclusion of HFP reduces crystalline fraction and ease of processing.
- VDF-TrFE: Can be more costly than PVDF-HFP because TrFE monomer is more expensive, and controlling ferroelectric properties requires tight compositional precision.
9. Summary of Key Differences
| Feature | Homopolymer PVDF | PVDF-HFP Copolymer | VDF-TrFE Copolymer |
|---|---|---|---|
| Primary Monomers | 100 % VDF | VDF + HFP (typically 5–20 mol % HFP) | VDF + TrFE (commonly 50–50 mol % to 70–30 mol %) |
| Crystallinity | High (≈ 45–55 %) | Moderate to Low (≈ 30–40 %) | Moderate (≈ 35–45 %), ferroelectric regions |
| Melting Point (Tₘ) | ≈ 170 °C | 140–160 °C | 135–160 °C (dependent on TrFE content) |
| Tensile Strength / Modulus | ≈ 35–50 MPa / 1.5–2.2 GPa | ≈ 20–35 MPa / 0.8–1.5 GPa | ≈ 25–40 MPa / 1.0–1.8 GPa |
| Elongation at Break | ≈ 50–100 % | ≈ 100–200 % | ≈ 100–150 % |
| Piezoelectric / Ferroelectric | Strong piezoelectric β-phase (d₃₃ ≈ −30 pC/N) | Weak piezoelectric, no strong ferroelectric | Moderate piezoelectric & intrinsic ferroelectric |
| Processability | Higher melt viscosity; narrower window | Lower viscosity; broader processing window | Lower viscosity; film casting for electronics |
| Chemical Resistance | Excellent | Excellent (slightly increased permeability) | Excellent (slightly lowered crystallinity) |
| Typical Uses | Sensors, actuators, chemical equipment, membranes | Battery binders, flexible coatings, dielectric elastomers | Ferroelectric memories, pyroelectric sensors, soft actuators |
| Relative Cost | High | Moderate to Low | Moderate to High |
10. Practical Selection Guidelines
- If high mechanical rigidity, maximum chemical resistance, or strong piezoelectric performance is required (e.g., ultrasonic transducers, chemical‐processing components), choose homopolymer PVDF.
- If flexibility, ease of processing (especially thin films), or use as a polymer matrix for battery or fuel cell applications is paramount, PVDF-HFP copolymer typically offers the best compromise between cost and performance.
- If intrinsic ferroelectric behavior and moderate piezoelectric properties are desired (e.g., ferroelectric random access memory, pyroelectric detectors, soft actuators), the VDF-TrFE copolymer is most suitable.
11. Conclusion
Although both homopolymer and copolymer PVDF share a common vinylidene fluoride backbone, the introduction of comonomers such as HFP or TrFE imparts significant changes in crystallinity, thermal behavior, mechanical flexibility, and electrical response.
Homopolymer PVDF excels in applications demanding high crystallinity, strong piezoelectricity, and exceptional chemical/thermal stability. Copolymers—PVDF-HFP and VDF-TrFE—provide tunable properties for flexible electronics, energy storage, and ferroelectric devices.
Selecting between homopolymer and a specific copolymer grade depends on balancing mechanical requirements, processing constraints, electrical properties, and overall cost considerations.